The locomotive model in essays 14 to 16 were non-vertebrate. Essay 17 takes the same problems, avoiding obstacles and seeking food, and with a model based on the vertebrate brain. Since these models are still Precambrian or early Cambrian, they don’t include the full vertebrate architecture, but try to find core components that might have been a basis for later vertebrate developments.
The animal is a slug-like creature with mucociliary forward movement, where propulsion is cilia or cilia-like and steering is muscular. This combination of slug-like motion and vertebrate brain is probably not evolutionary accurate, but it allows touch-based obstacle avoidance without the complications of vision of lateral-line senses.
The animal seeks food by following odor plumes, and avoids obstacles by turning away when touching them. The locomotion model includes the following components:
- [Braitenberg 1984] navigation (simple crossed vs uncrossed signals for approach and avoid).
- Obstacle avoidance with a direct touch-to-muscle circuit.
- Odor-seeking with distinct “what” and “where” paths.
- Perseveration fix with an explicit give-up circuit.
- Motivation-state (satiety) control of odor-seeking (“why” path [Verschure et al. 2014])
Proto-vertebrate model
A diagram of the proto-vertebrate model, including analogous brain regions follows:
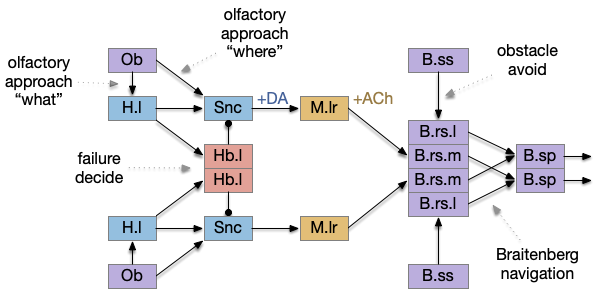
For the sake of readability, the model simplifies the actual vertebrate midline crossing patterns, leaving only a single cross between B.rs (reticulospinal) and B.sp (spinal), which represents Braitenberg navigation.
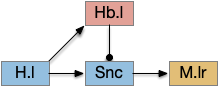
In this model, obstacle avoidance is reflexive between B.ss (somatosensory touch) and B.rs. Odor navigation (“where”) flows through Snc (substantia nigra pars compacta) to M.lr (midbrain locomotive region). In the zebrafish, the Snc area is the posterior tuberculum, and the M.lr like represents M.ppn (pedunculopontine tegmental nucleus). The motivation-state (hunger or satiety) and “what” (food odor vs non-food) flow through H.l (lateral hypothalamus). The give-up circuit flows through Hb.l (lateral habenula).
Olfactory navigation path
[Derjean et al. 2010] traced a path in zebrafish from Ob (olfactory bulb) to the posterior tuberculum (mammal Snc) to the midbrain locomotive region (likely M.ppn), to the reticulospinal motor command neurons.
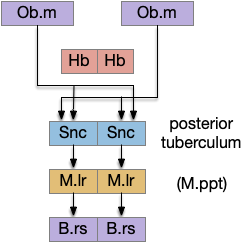
A similar olfactory to motor path has been traced in lamprey by [Suryanarayana et al. 2021] and [Beausejour et al. 2021].
I’ve labeled this path as a “where” path, based on simulation requirements, but as far as I know, that label has no scientific basis.
The Snc / posterior tubuculum area includes descending glutamate and dopamine (DA) neurons, although the Snc is better known for its ascending dopamine path. Since [Ryczko et al. 2016] reports a mammalian descending glutamate and DA path from Snc to M.ppn, portions of this descending path appears to be evolutionarily conserved. The DA appears to be an effort boost, increasing downstream activity, but most of the activity is glutamate.
Braitenberg navigation
[Braitenberg 1986] vehicles are a thought experiment for simple circuits to implement approach and avoid navigation. In the original, the vehicles have two light-detection sensors connected to drive wheels. Depending on the connection topology, sign and thresholds, the simple circuits can implement multiple behaviors.
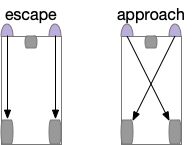
A circuit that combines the output of approach and avoid circuits with some lateral inhibition can implement both approach and avoidance with avoidance taking priority. In the essay simulation, if the animal touches a wall, it will turn away from the obstacle, temporarily ignoring any odor it might be following.
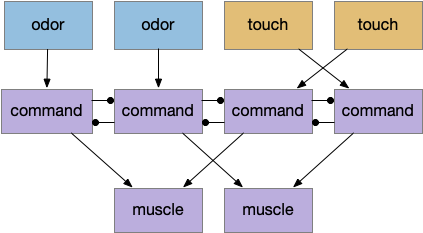
Mammalian locomotion appears to use a similar circuit between the superior colliculus (OT – optic tectum) and the motor driving B.rs neurons [Isa et al. 2021]. This circuit pattern implies that approach and avoidance are separate behaviors, only reconciled at the end. For example, a punishing reinforces that increases avoidance is not simply the mirror image of a non-reward that decreases approach. The two reinforcers modify different circuits.
“What” path vs “where” path
The mammalian visual system has separate “what” and “where” paths. One path detects what object is in focus, and one path keeps track of where the object location is. This division between object decision and navigation has been useful in the simulation, because navigation details are quickly lost in the circuit when deciding what to do with an odor.
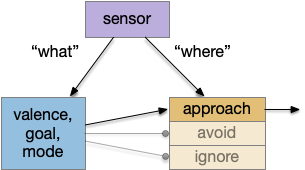
When an animal senses an odor, say a food odor, the animal needs to identify it as a food odor, decide if the animal is hungry or sated, and decide if there’s a higher-priority task. All that processing and decision can lost the fine timing phase and amplitude details needed for precise navigation. Gradient following, for example, needs fine differences in timing or amplitude to decide whether to turn left or right. By splitting the long, complicated “what” decision from the short, simple “where” location, the circuit can benefit from both.
[Cohn 2015] describes the fruit fly mushroom body as a switchboard, where dopamine neurons configure the path for olfactory senses to travel. In the context of “what” and “where”, the “what” path configures the switchboard and the “where” path follows the connected circuit.
Some odor-based navigation has a more extreme division between “what” and “where.” Following odor in water isn’t always gradient-based navigation, because odors form clumps instead of gradient plumes. Instead of following a gradient, the animal moves against the current toward the odor source. In that latter situation, the “where” path uses entirely different senses for navigation, using water flow mechanosensors, not olfactory sensors [Steele et al. 2023].
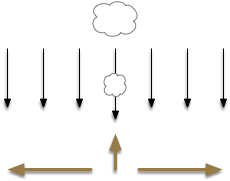
The diagram above illustrates a food-searching strategy for some animals in a current, both water and air. In water, the current is more reliable for navigation than an odor gradient. When there’s no scent, the animal swims back and forth across the current. When it detects a food odor, it swims against the current. If it loses the odor, it will return to back and forth swimming. In this navigation type, entirely different senses drive the “what” and “where” paths.
Foraging and give-up time
Giving up is an essential part of goal-directed behavior. If an animal cannot ever give up, it will be stuck on the goal without escaping. In the context of foraging, the give-up time is optimized with the marginal value theorem [Charnov 1976], suggesting that an animal should move to another patch when its current reward-gaining rate drops below the average rate for the environment. Animal behavior researchers like [Kacelnik and Brunner 2002] have observed animals roughly following this theorem, although using simpler heuristics.
In more complex animals, the failure to give up can be pathological, such as psychological perseveration.
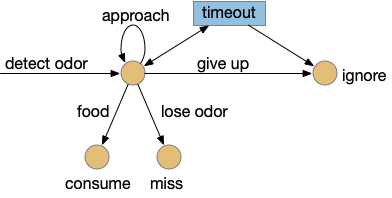
The give-up circuit needs some kind of internal timer or cost integrator, and a way to cancel the task. In this essay’s model, the lateral habenula (Hb.l) computes the give-up time or integrates the cost, and it cancels the task by suppressing the locomotive signal through Snc.
Habenula as a give-up circuit
Hb.l is positioned to act as a give-up circuit. It receives cost signals as non-rewarded bouts or as aversive events. [Stephenson-Jones et al. 2016] interprets the Hb.l input, P.hb (habenular-projecting pallidum), as evaluating action outcome. Hb.l can suppress both the midbrain dopamine and midbrain serotonin areas. In learned helplessness situations or depression, Hb.l is hyperactive [Webster et al. 2020], causing reduced activity.
[Hikosaka 2012] suggests the habenula’s role as suppressing motor activity under aversive conditions, a role evolved from its close relationship to the pineal gland’s circadian scheduling.
In a review article, [Hu 2020] discusses the suppressive effects of the habenula, also remarking on its role as a reward-prediction error. In particular, noting that H.l (lateral hypothalamus) to Hb.l is aversive. The Hu article also notes that Hb.l knock-out abolishes the error signal from reward omission, not an error signal from aversive (shock or obstacles).
Once the threshold is crossed, the Hb.l to Snc signal produces behavioral avoidance, reduced effort and depressive-like behavior from learned helplessness. The Hb.l is the only brain area consistently hyperactive in animal models of depression.
Note, since this essay’s simulation is a non-learning behavioral model, the only “prediction” possible is an evolutionary intrinsically-attractive odor, and the only role for an error is giving up the current behavior. Here, I’m interpreting the H.l to Hb.l signal as a cost signal, integrated by Hb.l, that gives up when it crosses a threshold.
Vertebrate reference
For reference, here’s a functional model of the vertebrate brain.
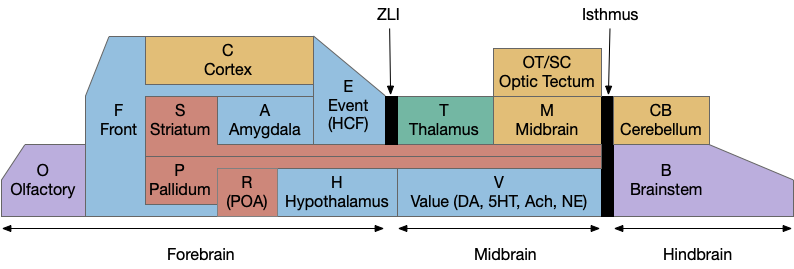
The areas in this model cluster around the hindbrain isthmus divider. B.rs are hindbrain neurons near the isthmus. M.lr (M.ppn) are midbrain neurons that migrate from the hindbrain (r1) to the midbrain. Snc is the midbrain tegmental area (the V – value area), near the isthmus, and contiguous with M.ppn. Similarly the H.l area that projects to Snc is contiguous with it. The habenula is the most distant area, located above the thalamus near the pineal gland (not in the diagram as a simplification, but associated with the pallidum areas.) So, the areas discussed here are a small part of the entire brain, but interestingly clustered around the isthmus divider near the cerebellum.
Minimal viable straw man
I think it’s important to remember that the essay simulations are an engineering project not a scientific one. One difference is that the simulations necessary require decisions beyond science. Another difference is that the project needs a simple core that may not correspond to any evolutionary animal. For example, even simple animals have some rudimentary vision, if only two or three pigment spots. For another, learning centers like the mushroom body. And dealing with internal biological issues like breathing and blood pressure with motion.
This model in particular is more of a straw man or minimal viable product than an actual proposal for an ancestral proto-vertebrate mind. The model is intended to be a straw man, a target that might give a base framework to criticize or build on.
Alternative olfactory paths
Another potential “what” path for innate behavior goes through the medial habenula, which is responsive to odors and produces place avoidance [Amo et al. 2014], but [Chen et al. 2019] suggests it also supports attraction for food odors.
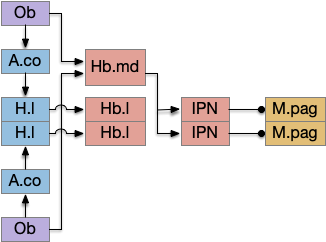
In mammals, the olfactory path to H.l goes through the cortical amygdala (A.co) [Cádiz-Moretti et al. 2017]. While this essay is deliberately omitting the cortex, in the lamprey the olfactory path goes through the lateral pallium (LPa, corresponding to mammalian O.pir piriform cortex) to the posterior tubercular (Snc in mammals.)
For this essay, I’ve picked the Ob to Snc path instead of the alternatives for simplicity. The habenula path is very tempting, but would require exploring the IPN and serotonin (5HT) paths to the MLR, which is more complicated than a “what” path through H.l
Subthalamic nucleus as give-up circuit
The sub thalamic nucleus (H.stn) is associated with a “stop” action, stopping downstream motor actions, either because of a new, surprising stimulus, or from higher-level commands. Since a give-up signal stops the seek goal, the stop action from H.stn might play a part in the control
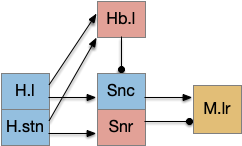
H.stn is believed to have a role in patience in decision making [Frank 2006] and in encoding reward and cost [Zénon et al. 2016], which is very similar to the role of the habenula, and H.stn projects to Hb.l via P.hb habenula-projecting pallidum.
However, the H.stn’s patience is more related to holding off (stopping) action before making a decision, related to impulsiveness, while the give-up circuit is more related to persistence, continuing an action. So, while the two capabilities are related, they’re different functions. Since current essay simulation does not have patience-related behavior arrest but does need a give-up time, the habenula seems a better fit.
Serotonin inhibition path
In zebrafish, the habenula inhibits the dorsal raphe (V.dr, serotonin neurons) but not Snc or dopamine [Okamoto et al. 2021]. The inhibition works through V.dr to the Snc/posterior tubuculum to the locomotive regions.
As with the alternative olfactory paths, this serotonin inhibition path may be more evolutionary primitive, but would add complexity to the essay’s model, so will be held off for later exploration.
Conclusions
As mentioned above, the purpose of this model is a basis for the current essay’s simulation, and as a straw man to focus alternatives to see if there might be a better minimal model.
References
Amo, Ryunosuke, et al. “The habenulo-raphe serotonergic circuit encodes an aversive expectation value essential for adaptive active avoidance of danger.” Neuron 84.5 (2014): 1034-1048.
Beauséjour PA, Zielinski B, Dubuc R. Olfactory-induced locomotion in lampreys. Cell Tissue Res. 2022 Jan
Braitenberg, V. (1984). Vehicles: Experiments in synthetic psychology. Cambridge, MA: MIT Press. “Vehicles – the MIT Press”
Cádiz-Moretti B, Abellán-Álvaro M, Pardo-Bellver C, Martínez-García F, Lanuza E. Afferent and efferent projections of the anterior cortical amygdaloid nucleus in the mouse. J Comp Neurol. 2017
Charnov, Eric L. “Optimal foraging, the marginal value theorem.” Theoretical population biology 9.2 (1976): 129-136.
Chen, Wei-yu, et al. “Role of olfactorily responsive neurons in the right dorsal habenula–ventral interpeduncular nucleus pathway in food-seeking behaviors of larval zebrafish.” Neuroscience 404 (2019): 259-267.
Cohn R, Morantte I, Ruta V. Coordinated and Compartmentalized Neuromodulation Shapes Sensory Processing in Drosophila. Cell. 2015 Dec 17
Derjean D, Moussaddy A, Atallah E, St-Pierre M, Auclair F, Chang S, Ren X, Zielinski B, Dubuc R. A novel neural substrate for the transformation of olfactory inputs into motor output. PLoS Biol. 2010 Dec 21
Frank, Michael J. “Hold your horses: a dynamic computational role for the subthalamic nucleus in decision making.” Neural networks 19.8 (2006): 1120-1136.
Hikosaka, Okihide. The habenula: from stress evasion to value-based decision-making. Nature reviews neuroscience 11.7 (2010): 503-513.
Hu, Hailan, Yihui Cui, and Yan Yang. “Circuits and functions of the lateral habenula in health and in disease.” Nature Reviews Neuroscience 21.5 (2020): 277-295.
Isa, Tadashi, et al. “The tectum/superior colliculus as the vertebrate solution for spatial sensory integration and action.” Current Biology 31.11 (2021)
Kacelnik, Alex, and Dani Brunner. “Timing and foraging: Gibbon’s scalar expectancy theory and optimal patch exploitation.” Learning and Motivation 33.1 (2002): 177-195.
Okamoto H, Cherng BW, Nakajo H, Chou MY, Kinoshita M. Habenula as the experience-dependent controlling switchboard of behavior and attention in social conflict and learning. Curr Opin Neurobiol. 2021 Jun;68:36-43. doi: 10.1016/j.conb.2020.12.005. Epub 2021 Jan 6. PMID: 33421772.
Ryczko D, Cone JJ, Alpert MH, Goetz L, Auclair F, Dubé C, Parent M, Roitman MF, Alford S, Dubuc R. A descending dopamine pathway conserved from basal vertebrates to mammals. Proc Natl Acad Sci U S A. 2016 Apr 26
Steele TJ, Lanz AJ, Nagel KI. Olfactory navigation in arthropods. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2023
Stephenson-Jones M, Floros O, Robertson B, Grillner S. Evolutionary conservation of the habenular nuclei and their circuitry controlling the dopamine and 5-hydroxytryptophan (5-HT) systems. Proc Natl Acad Sci U S A. 2012 Jan 17;109(3)
Stephenson-Jones M, Yu K, Ahrens S, Tucciarone JM, van Huijstee AN, Mejia LA, Penzo MA, Tai LH, Wilbrecht L, Li B. A basal ganglia circuit for evaluating action outcomes. Nature. 2016 Nov 10
Suryanarayana SM, Pérez-Fernández J, Robertson B, Grillner S. Olfaction in Lamprey Pallium Revisited-Dual Projections of Mitral and Tufted Cells. Cell Rep. 2021 Jan 5
Verschure PF, Pennartz CM, Pezzulo G. The why, what, where, when and how of goal-directed choice: neuronal and computational principles. Philos Trans R Soc Lond B Biol Sci. 2014 Nov 5
Webster JF, Vroman R, Balueva K, Wulff P, Sakata S, Wozny C. Disentangling neuronal inhibition and inhibitory pathways in the lateral habenula. Sci Rep. 2020 May 22
Zénon A, Duclos Y, Carron R, Witjas T, Baunez C, Régis J, Azulay JP, Brown P, Eusebio A. The human subthalamic nucleus encodes the subjective value of reward and the cost of effort during decision-making. Brain. 2016 Jun;139(Pt 6):1830-43.
