Although the essays have implemented obstacle avoidance, they haven’t yet explored olfactory avoidance. Olfactory avoidance is distinct from obstacles, not just because obstacles have higher priority, but because the olfactory system is from an entirely different nervous system than the sensorimotor system. In the chimaeral brain theory [Tosches and Arendt 2013], bilaterian brains are composed of an apical nervous system (ANS) focused on chemo senses (olfactory external and hypothalamic internal), and a blastoporal nervous system (BNS) focused on sensorimotor control like obstacle avoidance.
Olfactory path
The paths for olfactory motion compared with obstacle motion shows the value of the chimaeral theory in making sense of the brain. Working backward from the midbrain locomotive region (MLR), the acetylcholine (ACh) MLR nuclei specialize: the pedunculopontine nucleus (M.ppt) supports the sensorimotor BNS, and the laterodorsal tegmental nucleus (M.ldt) supports the chemosensory ANS.
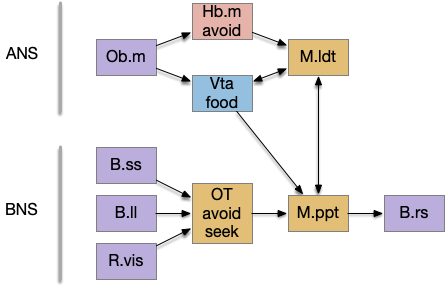
In the above diagram, food odors and warning odors use distinct paths to the MLR. Food odors from the olfactory bulb (Ob) pass through the ventral tegmental area (Vta – posterior tuberculum in zebrafish) to the MLR [Derjean et al. 2010]. Aversive odors like cadaverine pass through the medial habenula (Hb.m) to the M.ldt portion of the MLR [Stephenson-Jones et al. 2012]. The food and avoidance paths are distinct because hunger and satiety from the hypothalamus modulate the food path, while the avoidance path can pass through unmodulated. These olfactory locomotion paths correspond to the ANS.
Lamprey medial habenula path
All vertebrates share this basic architecture, including the lamprey, one of the most evolutionary-distant vertebrates. [Stephenson-Jones et al. 2012] traced the Hb.m circuit, showing that Hb.m inputs are from the olfactory path, the parapineal (light attraction), and an electron-sensory alarm to the interpeduncular nucleus (M.ip).
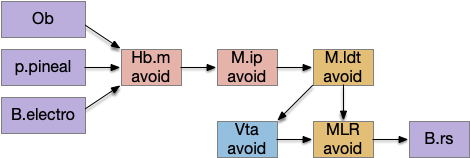
The above diagram fills out the olfactory warning path. The interpeduncular nucleus is a key node in the avoidance circuit, and also key to locomotor-induced theta, and one of the two serotonin nodes. Mip has a major output to the serotonin areas: dorsal raphe (V.dr) and medial raphe (V.mr) and to the central grey (M.pag) [Quina et al. 2017] and M.ldt as well as structures associated with hippocampal (E.hc) theta [Lima et al. 2017].
Medial habenula behavior
In larval zebrafish, Hb.m supports olfactory avoidance [Choi et al. 2017], [Jeong et al. 2021], and light seeking [Zhang et al. 2017]. At least one study indicates that it may also affect food seeking [Chen et al. 2019]. The non-Ob input to Hb.m — the posterior septum (P.ps) — produce locomotion when stimulated [Ostu et al. 2018], suggesting that later evolved functionality maintains the original basal function.
In zebrafish, M.ip only projects to serotonin areas (V.dr and V.mr), not to dopamine or MLR areas. The lamprey connectivity suggests that the M.ip to M.ldt connection was lost in fish.
The Hb.m to M.ip connection is affected by nicotine. An interesting property is that low stimulation and high stimulation have opposite effects. Low stimulation uses glutamate connections and is attractive while high stimulation adds ACh and is aversive [Krishnan et al. 2014].
Developmental genetic notes
As an interesting aside, both Hb.m and avoidant layers of OT shared a genetic marker Brn3a (aka pou4f1) [Quina et al. 2009], [Fedtsova et al. 2008]. That marker also appears in the cerebellum’s inferior olive, trigeminal sensory areas, and the amphioxus motor LPN3 neuron [Bozzo et al. 2023].
M.ldt and M.ppt are sibling areas, deriving from the r1 rhombic lip [Machold et al. 2011].
Glutamate and GABA neurons in M.ip, Vta, and M.ldt all derive from r1 basal neurons [Lahti et al. 2016].
Locomotion switchboard
The addition of olfactory avoidance further complicates the switchboard combining the various locomotor streams, especially if the olfactory path uses serotonin as a modulator as opposed to a straight glutamate connection. Although I’ll probably use a fixed priority for essay 20, and as [Cisek 2022] notes, avoidance can be combined additively, at some point the switchboard will need more control, especially when essays add vision and consummatory actions.
References
Bozzo M, Bellitto D, Amaroli A, Ferrando S, Schubert M, Candiani S. Retinoic Acid and POU Genes in Developing Amphioxus: A Focus on Neural Development. Cells. 2023 Feb 14
Chen W-Y, Peng X-L, Deng Q-S, Chen M-J, Du J-L, Zhang B-B. Role of Olfactorily Responsive Neurons in the Right Dorsal Habenula-Ventral Interpeduncular Nucleus Pathway in Food-Seeking Behaviors of Larval Zebrafish. Neuroscience. 2019
Choi JH, Duboue ER, Macurak M, Chanchu JM, Halpern ME. Specialized neurons in the right habenula mediate response to aversive olfactory cues. Elife. 2021 Dec 8
Cisek P. Evolution of behavioural control from chordates to primates. Philos Trans R Soc Lond B Biol Sci. 2022 Feb 14
Derjean D, Moussaddy A, Atallah E, St-Pierre M, Auclair F, Chang S, Ren X, Zielinski B, Dubuc R. A novel neural substrate for the transformation of olfactory inputs into motor output. PLoS Biol. 2010 Dec 21
Fedtsova N, Quina LA, Wang S, Turner EE. Regulation of the development of tectal neurons and their projections by transcription factors Brn3a and Pax7. Dev Biol. 2008 Apr 1
Jeong YM, Choi TI, Hwang KS, Lee JS, Gerlai R, Kim CH. Optogenetic Manipulation of Olfactory Responses in Transgenic Zebrafish: A Neurobiological and Behavioral Study. Int J Mol Sci. 2021 Jul 3
Krishnan S, Mathuru AS, Kibat C, Rahman M, Lupton CE, Stewart J, Claridge-Chang A, Yen SC, Jesuthasan S. The right dorsal habenula limits attraction to an odor in zebrafish. Current Biology. 2014
Lahti L, Haugas M, Tikker L, Airavaara M, Voutilainen MH, Anttila J, Kumar S, Inkinen C, Salminen M, Partanen J. Differentiation and molecular heterogeneity of inhibitory and excitatory neurons associated with midbrain dopaminergic nuclei. Development. 2016 Feb 1
Lima LB, Bueno D, Leite F, Souza S, Gonçalves L, Furigo IC, Donato J Jr, Metzger M. Afferent and efferent connections of the interpeduncular nucleus with special reference to circuits involving the habenula and raphe nuclei. J Comp Neurol. 2017 Jul 1
Machold R, Klein C, Fishell G. Genes expressed in Atoh1 neuronal lineages arising from the r1/isthmus rhombic lip. Gene Expr Patterns. 2011 Jun-Jul
Otsu Y, Lecca S, Pietrajtis K, Rousseau CV, Marcaggi P, Dugué GP, Mailhes-Hamon C, Mameli M, Diana MA. Functional Principles of Posterior Septal Inputs to the Medial Habenula. Cell Rep. 2018 Jan 16
Quina LA, Wang S, Ng L, Turner EE. Brn3a and Nurr1 mediate a gene regulatory pathway for habenula development. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2009
Stephenson-Jones M, Floros O, Robertson B, Grillner S. Evolutionary conservation of the habenular nuclei and their circuitry controlling the dopamine and 5-hydroxytryptophan (5-HT) systems. Proc Natl Acad Sci U S A. 2012 Jan 17
Tosches, Maria Antonietta, and Detlev Arendt. “The bilaterian forebrain: an evolutionary chimaera.” Current opinion in neurobiology 23.6 (2013): 1080-1089.
Zhang BB, Yao YY, Zhang HF, Kawakami K, Du JL. Left Habenula Mediates Light-Preference Behavior in Zebrafish via an Asymmetrical Visual Pathway. Neuron. 2017 Feb 22
