Essay 18 was trying to solve the problem of maintaining behavioral state. When a fast neuron synapse takes only 5ms, behavior that lasts seconds or minutes needs some circuit to sustain attention on the task. Essay 18 explored the striatum as a possible model to maintain behavior. In zebrafish, this problem is partial solved with a paired system consisting of the optic tectum (OT) and the nucleus isthmi (NI) [Gruberg et al. 2006].
Optic tectum
The optic tectum (OT – superior colliculus in mammals) is a midbrain action and sensor system that organizes vision, touch, sound, and action into retinotopic map like an air controller radar screen that activates only for important triggers. So, it’s not like the movie screen of primate vision, but is an action-oriented, sparse map that focuses on a few important items. In the larva zebrafish, the OT activates for hunting prey (paramecia) and avoiding obstacles and predators.
The OT itself has no persistence, When it detects potential prey and moves toward the prey, the OT doesn’t remember that it’s hunting or recall the previous location of the prey. Without enhancement, it forgets the pretend fails the hunt. The nucleus isthmi (NI – parabigeminal in mammals) provides that attention and persistence function [Henriques et al. 2019].
Nucleus isthmi circuit
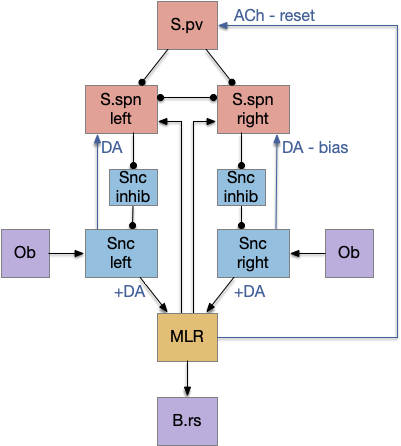
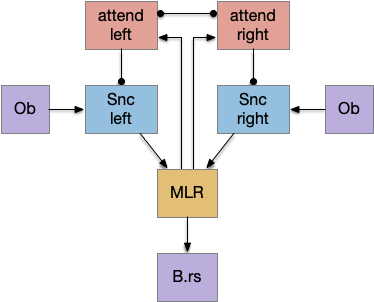
The NI has a simple organization that is topologically, bidirectionally mapped to OT. The return signal from NI to OT is acetylcholine (ACh), which amplifies the sense input, biasing the next action to follow the previous action. Essentially this is a simple attention circuit that maintains consistent behavior.
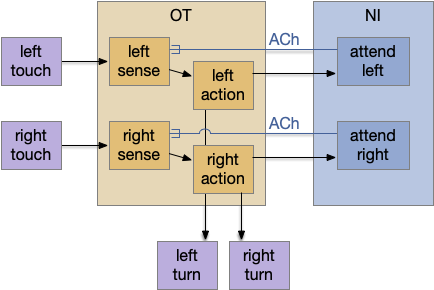
In the diagram above, a left action sends an efference copy to the matching nucleus isthmi area, which can remember the activation for longer than the 5ms fast activation in the OT. In turn it sends an ACh modulator to amplify the left touch sensor, biasing the direction toward the same action.
For the essay simulation, the original problem was hitting an obstacle head-on, which triggered both left and right touch sensors, which then caused jitter as the animal randomly chose left and right without maintaining consistency. By adding an NI system, an initial left action would bias the left input sense to choose a next left action.
Acetylcholine attention system
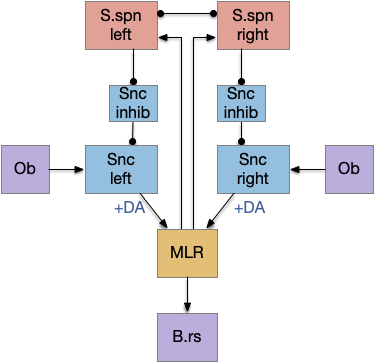
As a speculation, or perhaps a mnemonic, this NI system where ACh enhances senses based on action might be a model for some attention mechanisms else were in the brain. NI is a sister nucleus to other ACh nuclei, specifically the parabrachial nucleus (B.pb) and the pedunculopontine nucleus (V.ppn), all developing from the same stem region near the isthmus. V.ppn is one of the major ACh attention nuclei and is part of the midbrain locomotive region (MLR). It seems plausible that V.ppn might share some organization with NI where its upstream ACh might support sense attention like the NI does for OT.
Engineering note
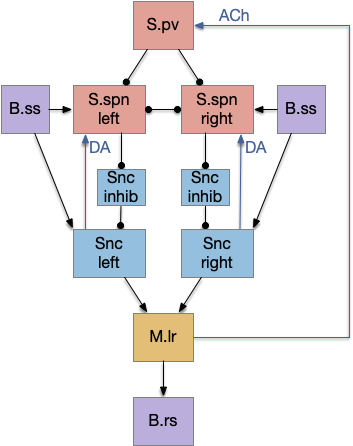
After implementing the nucleus isthmi support, both the proto-striatum and NI solve the jittering problem equally. The algorithms are slightly different — NI is a straight enhancement, while proto-striatum is a disinhibition with selection — but for the current complexity of the animal and environment, there’s no behavioral difference. Both proto-striatum and NI can be enabled simultaneously without interference problems.
References
Cui H, Malpeli JG. Activity in the parabigeminal nucleus during eye movements directed at moving and stationary targets. J Neurophysiol. 2003 Jun;89(6):3128-42. doi: 10.1152/jn.01067.2002. Epub 2003 Feb 26
Gruberg E., Dudkin E., Wang Y., Marín G., Salas C., Sentis E., Letelier J., Mpodozis J., Malpeli J., Cui H. Influencing and interpreting visual input: the role of a visual feedback system. J. Neurosci. 2006
Henriques PM, Rahman N, Jackson SE, Bianco IH. Nucleus Isthmi Is Required to Sustain Target Pursuit during Visually Guided Prey-Catching. Curr Biol. 2019 Jun 3
Marín G, Salas C, Sentis E, Rojas X, Letelier JC, Mpodozis J. A cholinergic gating mechanism controlled by competitive interactions in the optic tectum of the pigeon. J Neurosci. 2007 Jul 25
Motts SD, Slusarczyk AS, Sowick CS, Schofield BR. Distribution of cholinergic cells in guinea pig brainstem. Neuroscience. 2008 Jun 12;154(1):186-95. doi: 10.1016/j.neuroscience.2007.12.017. Epub 2008 Jan 28