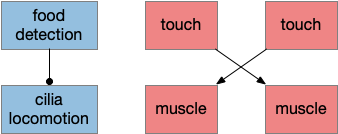
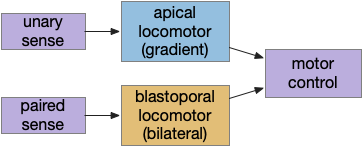
Although the previous essays have focused on bilateral locomotion in the style of Braitenberg machines, the chimaera brain hypothesis [Tosches and Adrendt 2013] suggests a distinct apical form of locomotion. The chimaera brain hypothesis suggests that bilaterian brains are the merger of an apical nervous system from the ancestral zooplankton larva state and a blastoporal (bilateral) nervous system from paired muscles along the spinal cord. The apical area contains unpaired light and chemical sensors and the blastoporal area contains bilateral, topographic somatic sense like touch. Apical navigation require a temporal gradient, calculated by sequential sampling because the apical senses are non-directional.
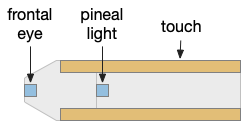
The essays’ simulation scenario is a temporal phototaxis based on the real-time place preference (RTPP) experiments of [Chen et al 2014], where a zebrafish stayed inside a virtual light circle, avoiding surrounding dark area. Temporal phototaxis is gradient-based locomotion, heading toward light and away from darkness by comparing light samples at different times.
Chimaera locomotor
The vertebrate paired eye and paired olfaction are late vertebrate developments. The pre-vertebrate animal amphioxus has only a single frontal eye. Since pre-paired sense animals needed to navigate toward opportunities and away from threats, it’s conceivable that apical random-walk navigation developed before visual navigation.
Apical gradient
The apical area contains undirected light and chemical sensors. The apical area is based on the zooplankton larval state as shown below, while the bilateral area is based on bilateral worm-like adults structured like the spinal cord of paired muscles and neurons.
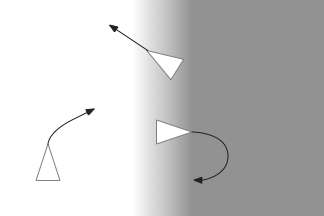
Apical navigation requires following a temporal gradient, calculated by sequential sampling. While bilateral areas can compare left and right senses to calculate a spatial gradient, the single apical sense is restricted to a temporal gradient.
Bacteria tumble and run
Even simple bacteria can follow gradients using a directed random walk strategy called tumble and run [Segall et al 1986]. The bacteria’s flagella have two modes: tumble, turning without moving, and run, moving forward without turning. By alternating tumble with run, the bacteria can search with a random walk. By extending the run phase when the gradient improves, the bacteria can move toward the target.
Importantly, a temporal gradient calculation needs some sort of memory, accumulator or integrator to compare the current value to recent values. In bacteria, tis accumulator is an internal chemical quantity.
Apical and bilateral sensors
Amphioxus is a pre-vertebrate chordate that’s studied to understand vertebrate evolution. Amphioxus does not have a paired eye, instead it has a single frontal eye that amphioxus uses to orient vertically, and also a pineal-like photoreceptor [Lacalli 2020].

The pineal region is near the vertebrate habenula. Amphioxus does not have a habenula, but it does have a nearby motor control neuron, LPN3, with similar genetic markers [Bozzo et al 2023]. Modern vertebrate medial habenula receives undirected light input from the retina, but it seems plausible that an early habenula used the pineal photosensor because both are part of the same epithalamus complex, and only later connected to the newly-paired retina when it developed.
Multiple apical regions
Before diving into the vertebrate areas for apical locomotion, I need to explain why widespread areas can all be apical, including midbrain and hindbrain areas. Vertebrates had two rounds of whole genome duplication [Dehal and Moore 2005], which gives an easy evolutionary opportunity for four apical areas from the genome duplication, in addition to other possible duplications. The xenobot experiments [Blackiston et al 2023] shows that biology can mix multiple copies like the split apical area into a coherent animal: development can be flexible.
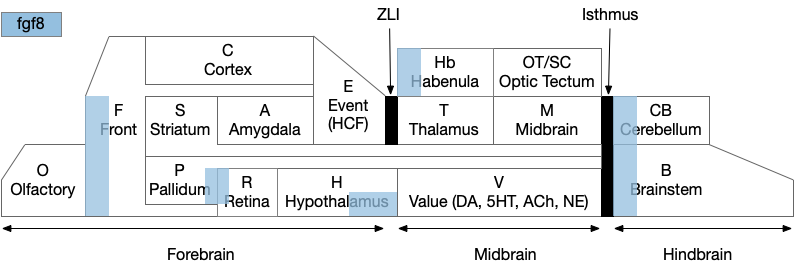
The diagram above is a functional representation of the vertebrate brain with possible apical areas highlighted in blue. Fgf8 is a development growth factor associated with the apical area [Marlow et al 2014]. The four (or five) possible apical area are as follows:
- Prefrontal cortex and olfactory bulb.
- The caudal isthmus area (r1) at the midbrain-hindbrain boundary, including cerebellum (CB), interpeduncular nucleus (M.ip), midbrain locomotor (MLR, M.ppt, M.ldt), head direction (B.dtg), parabrachial (B.pb), and part of the substantia nigra (Snr).
- Habenula (Hb) and pre-thalamic eminence area (P.em) near ZLI. Also, between the septum-diagonal band (P.msdb) and preoptic area (Poa) near the optic (retina) region (R).
- The mammilary and supramammilary area of the hypothalamus (H.mb and H.sum).
I’ve listed four regions instead of the five blue areas because the Hb-P.em-P.msdb area are physical closer than the diagram suggests, are split more by the alar/basal division than distance, and is complicated by distortions from the paired optic region.
This essay uses functions from the r1 isthmus area (M.ip and MLR.α / M.ldt), and from the habenula / retinal area (Hb.m, P.em, pineal, and retina). The logic behind their connectivity is a function split-and-pull like taffy or continental drift from a single pre-duplication area, for example, the isthmus apical area duplicating from an original pre-hypothalamus / retinal apical area.
As a note, the supramammilary area (H.sum) is highly connected with the area in this essay, but I’m postponing exploring its functionality for now.
Vertebrate apical and bilateral locomotor
Previously the essays used the bilateral locomotor path, going through the Vta (posterior tuberculum), tectum (OT), and midbrain locomotive region (MLR). The apical path runs through the medial habenula (Hb.m) and the interpeduncular nucleus (M.ip) before reaching the motor neurons.
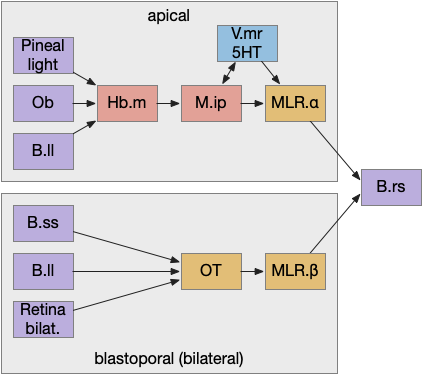
The lamprey’s Hb.m supports locomotion for light, odor, and the lateral line [Stephenson-Jones et al 2012]. The lateral line is an aquatic sense for water flow, which allows fish to sense nearby objects. Although the exact functional division of phototaxis isn’t known, Hb.m, M.ip and the serotonin raphe nuclei (V.mr – 5HT) are all required [Cheng et al 2016].
For the essay’s simulation, I’m asking the integration and running average to the V.mr and 5HT, but this is something of a guess, because phototaxis integration hasn’t been measured. In the essay’s model, M.ip translates the light data and 5HT average into a gradient and into action.
Phototaxis actions
When zebrafish enter darkness from light, they immediately produce a large turn (O-bend) and start an area restricted search (ARS) [Fernandes et al 2012]. Later turns are smaller [Chen and Engert 2014].
For the essay, phototaxis gradient modifies the standard random walk. When entering darkness, M.ip increases the turn angle. When entering light, M.ip increases forward movement, extending the run.
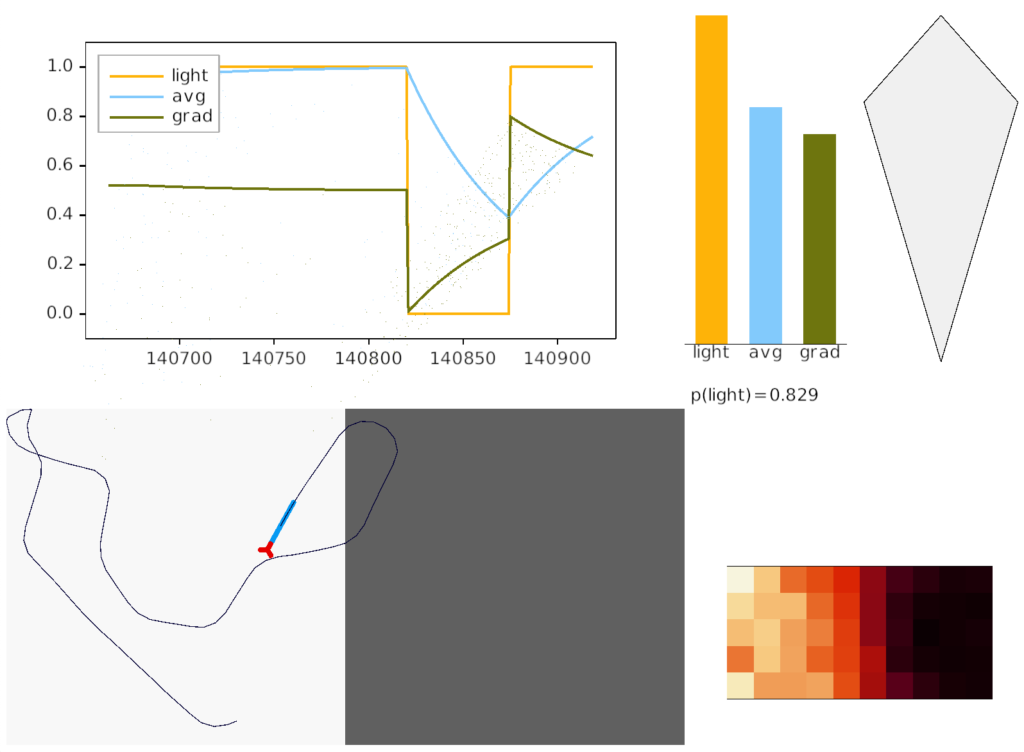
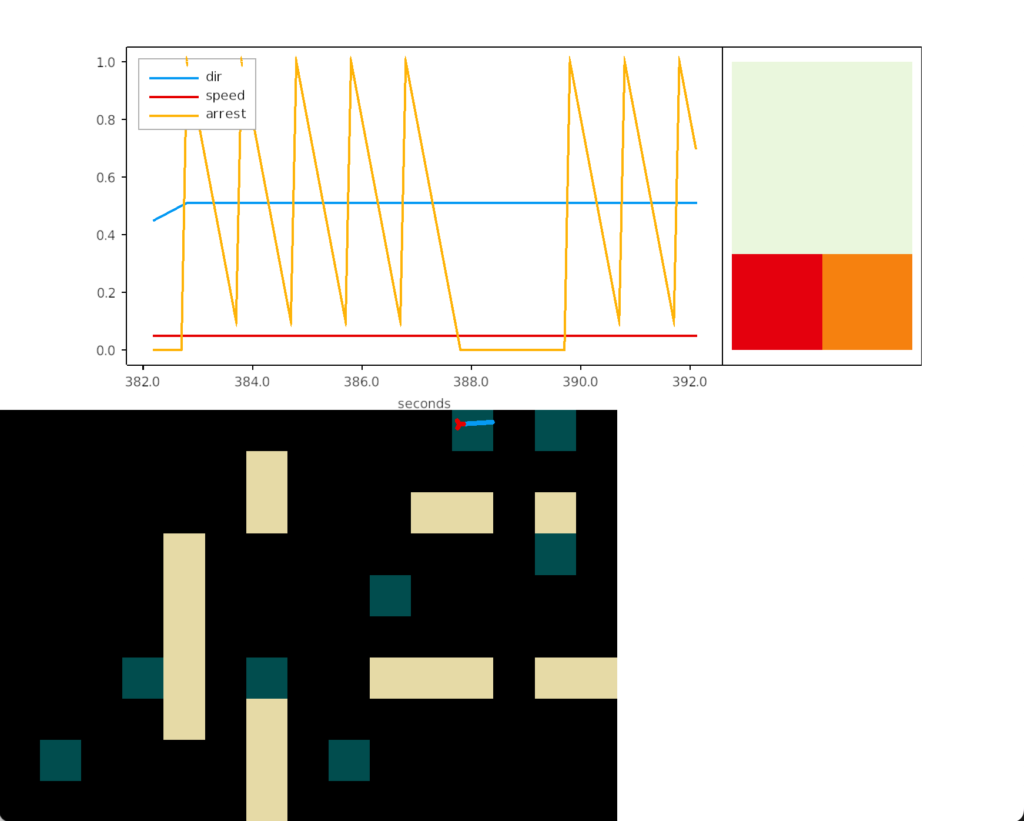
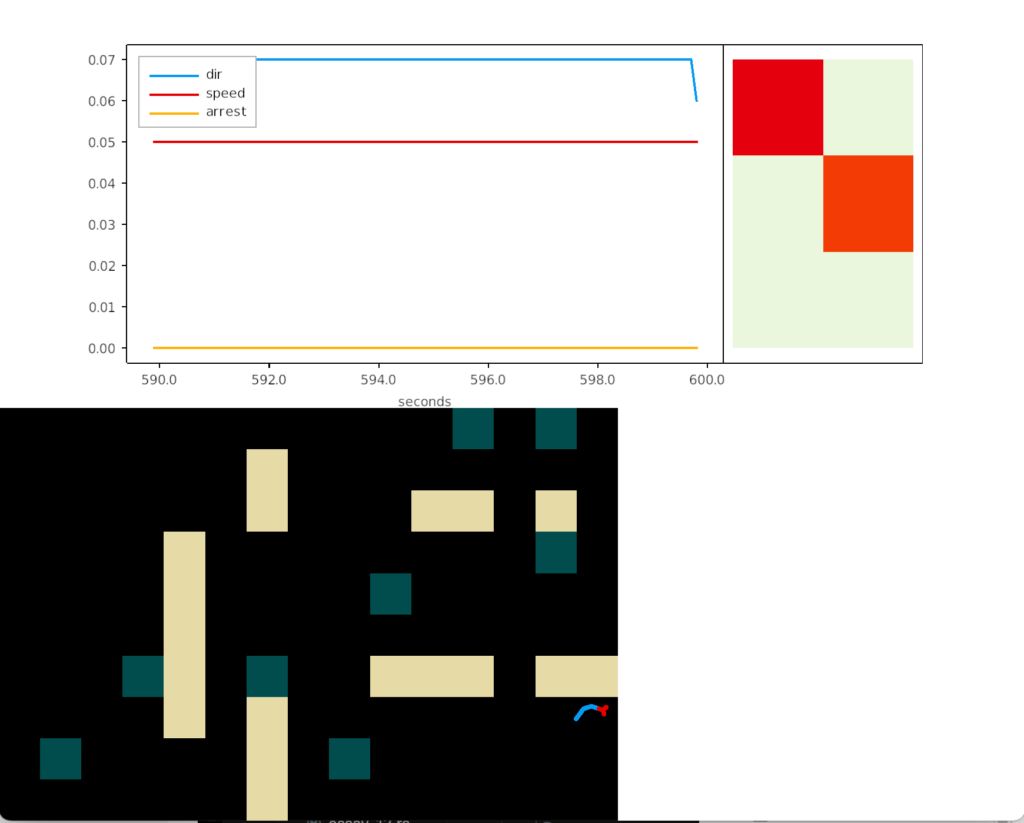
The above screenshot shows the animal crossing into darkness and returning to light. In the graph, “light” is the current photosensor value, “avg” is the running average measured by serotonin neurons, and “grad” is the different between the two. A gradient drop triggers high angle turns. A gradient rise triggers straight movement.
As the heat map in the right shows, this simple system produces real-time place avoidance (RTPA) of darkness. Since the system has no learning, there’s no conditioned place aversion (CPA).
Prethalamic eminence
The full phototaxis circuit in vertebrates is a bit more complicated because light input does through an intermediate area called the pre-thalamic eminence (P.em), which is between the habenula, hypothalamus and thalamus, and it one of the apical areas. Although P.em is not cortical, it provides neurons necessary for cortical development (Cajal-Retzius neurons for L1 patterning) [Marin-Padilla 2015] and neurons for habenula input, the habenula-projecting pallidum (P.hb) [Stephenson-Jones 2016].
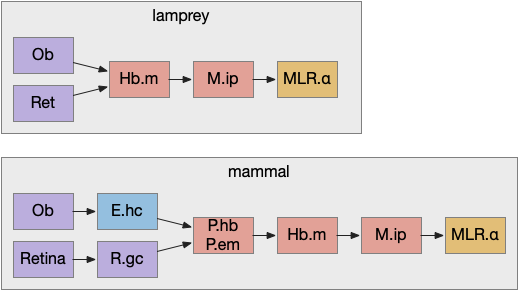
Retina input goes through P.em to Hb.m for phototaxis. Interestingly, the main input for Hb.m in mammals is via cells that migrate from P.em and become the posterior septum (P.ps) [Watanabe et al 2018], which receives almost all of its input from the hippocampus (E.hc). If the hippocampus is an odor-processing system, then the olfactory bulb (Ob) to E.hc to Hb.m path is a chemotaxis path matching the retina’s phototaxis path.
Note that the olfactory placed develops from the lens placed and is differentiated by fgf8 [Bailey et al 2006]. So, it’s pleasing that the similar olfactory and hippocampal paths to Hb.m is a are chemotaxis and phototaxis paths split from a common ancestor.
Speculation
Although the lateral and medial habenula are chemically, connectional, and developmentally distinct, their broad similarity is interesting. If the medial habenula supports direct, concrete sensory navigation by gradient descent, perhaps the medial habenula supports more abstract value-based navigation for more abstract goals.
References
Alonso A, Trujillo CM, Puelles L. Quail-chick grafting experiments corroborate that Tbr1-positive eminential prethalamic neurons migrate along three streams into hypothalamus, subpallium and septocommissural areas. Brain Struct Funct. 2021 Apr;226(3):759-785.
Bailey AP, Bhattacharyya S, Bronner-Fraser M, Streit A. Lens specification is the ground state of all sensory placodes, from which FGF promotes olfactory identity. Dev Cell. 2006 Oct;11(4):505-17.
Blackiston D, Kriegman S, Bongard J, Levin M. Biological Robots: Perspectives on an Emerging Interdisciplinary Field. Soft Robot. 2023 Aug;10(4):674-686.
Bozzo M, Bellitto D, Amaroli A, Ferrando S, Schubert M, Candiani S. Retinoic Acid and POU Genes in Developing Amphioxus: A Focus on Neural Development. Cells. 2023 Feb 14;12(4):614.
Chen X, Engert F. Navigational strategies underlying phototaxis in larval zebrafish. Front Syst Neurosci. 2014 Mar 25;8:39.
Cheng RK, Krishnan S, Jesuthasan S. Activation and inhibition of tph2 serotonergic neurons operate in tandem to influence larval zebrafish preference for light over darkness. Sci Rep. 2016 Feb 12;6:20788.
Dehal P, Boore JL. Two rounds of whole genome duplication in the ancestral vertebrate. PLoS Biol. 2005 Oct;3(10):e314.
Fernandes A. M., Fero K., Arrenberg A. B., Bergeron S. A., Driever W., Burgess H. A. (2012). Deep brain photoreceptors control light-seeking behavior in zebrafish larvae. Curr. Biol. 22, 2042–2047
Lacalli Thurston 2022 An evolutionary perspective on chordate brain organization and function: insights from amphioxus, and the problem of sentience Phil. Trans. R. Soc.
Marín-Padilla M. Human cerebral cortex Cajal-Retzius neuron: development, structure and function. A Golgi study. Front Neuroanat. 2015 Feb 27;9:21.
Marlow, Heather, et al. “Larval body patterning and apical organs are conserved in animal evolution.” BMC biology 12.1 (2014): 1-17.
Segall J. E., Block S. M., Berg H. C. (1986). Temporal comparisons in bacterial chemotaxis. Proc. Natl. Acad. Sci. U.S.A. 83, 8987–8991
Stephenson-Jones M, Floros O, Robertson B, Grillner S. Evolutionary conservation of the habenular nuclei and their circuitry controlling the dopamine and 5-hydroxytryptophan (5-HT) systems. Proc Natl Acad Sci U S A. 2012 Jan 17;109(3):E164-73.
Stephenson-Jones M, Yu K, Ahrens S, Tucciarone JM, van Huijstee AN, Mejia LA, Penzo MA, Tai LH, Wilbrecht L, Li B. A basal ganglia circuit for evaluating action outcomes. Nature. 2016 Nov 10;539(7628):289-293.
Tosches, Maria Antonietta, and Detlev Arendt. “The bilaterian forebrain: an evolutionary chimaera.” Current opinion in neurobiology 23.6 (2013): 1080-1089.
Watanabe K, Irie K, Hanashima C, Takebayashi H, Sato N. Diencephalic progenitors contribute to the posterior septum through rostral migration along the hippocampal axonal pathway. Sci Rep. 2018 Aug 6;8(1):11728.
Wolf S, Dubreuil AM, Bertoni T, Böhm UL, Bormuth V, Candelier R, Karpenko S, Hildebrand DGC, Bianco IH, Monasson R, Debrégeas G. Sensorimotor computation underlying phototaxis in zebrafish. Nat Commun. 2017 Sep 21;8(1):651.
Zhang BB, Yao YY, Zhang HF, Kawakami K, Du JL. Left Habenula mediates light-preference behavior in Zebrafish via an asymmetrical visual pathway. Neuron. 2017;93:914–28.