I’ve been ignoring distracting cues in the previous essays for simplification. Since the simulated animal only encountered a single odor at a time, it never needed to select one and ignore the other. In essay 26, I’ll implement a very simple first approximation to ignoring distractors, using the P.bf (basal forebrain) control of the Ob (olfactory bulb) as a switchboard to let the selected odor through and inhibit the ignored distractor.
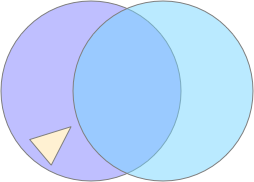
In the diagram above, the animal (triangle) is seeking food using the purple odor cue as a gradient direction. When it encounters the distractor odor in blue, it should ignore the distractor, otherwise the two odors will mingle into an incorrect summed gradient and the animal will seek in the wrong direction [Cisek 2022].
Temporal chemotaxis
For essay 26, I’m switching chemotaxis (odor seeking) to use the apical temporal gradient search, using Hb.m (medial habenula) and B.ip (interpeduncular nucleus) like the phototaxis in essay 24. The apical system follows the chimera brain model of [Tosches and Arendt 2013], which suggests that odor senses and actions are distinct systems from bilateral tactile senses. For the essays, the shift is from a bilateral, Braitenberg-like [Braitenberg 1984] system to a modulated random walk like the bacterial tumble-and-run.
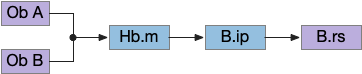
The above diagram shows the problem with distractor odors. Because the tumble-and-run system uses a single temporal gradient, it necessarily adds both odors together for its input. The summed input goes to the Hb.m (medial habenula) and B.ip (interpeduncular nucleus) system to modulate the random walk direction.
When the animal crosses into the overlapping distractor odor, it will follow the combined signal, distracted from the original seek target. To avoid distraction, the system can either amplify the current odor A, or inhibit the distractors like odor B.
Analogy with nucleus isthmi
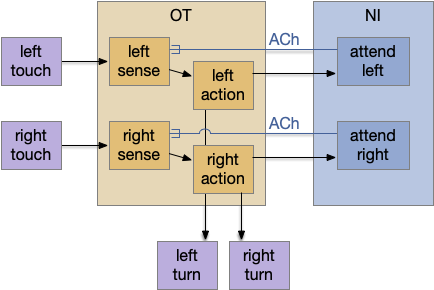
An earlier essay 19 also had an attention / distractor problem, with a different issue of action consistency, and used a zebrafish circuit in P.ni (nucleus isthmi) as a solution. In larval zebrafish P.ni works together with OT (optic tectum) to sustain attention on prey during a hunt [Henriques et al 2019]. P.ni is an ACh (acetylcholine neurotransmitter) and GABA (inhibiting neurotransmitter) system that both amplifies the predicted prey location and inhibits surrounding areas.
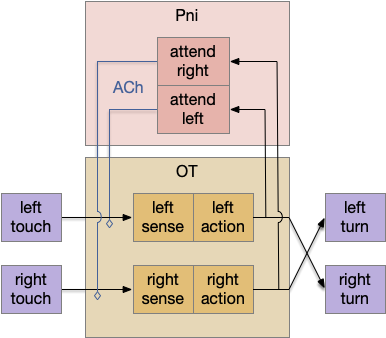
In the above diagram for the essay 19 circuit, a simultaneous left and right touch would select one action at random and sustain that choice for subsequent movement with the P.ni positive feedback circuit. The outputs are crossed because it’s an avoidance circuit: an obstacle on the left triggers a right turn.
Importantly, the positive feedback is modulatory; it doesn’t trigger an action by itself. At a synapse level, ACh triggers mAChR (ACh metabotropic receptor, Gs stimulatory type) on the sensor axon, amplifying the sensor’s neurotransmitter release. The ACh and mAChR act as the decay timer, because they have a slow time constant on the order of a few seconds. If the sensor doesn’t stimulate the circuit, as when successfully avoiding the obstacle, the attention will decay over a few seconds, resetting the system to its original state.
A similar function applies to Ob and P.bf (basal forebrain), where P.bf acts like P.ni to sustain attention to the selected odor. “Basal forebrain” is a general name for a collection of functionally-related subcortical areas in the ventral (“basal”) forebrain, all pallidal-like (P). The specific P areas for the Ob are P.hdb (horizontal diagonal band) and Po.me (magnocellular preoptic area), but I’ll use P.bf for simplicity.
Olfactory bulb as a switchboard
In this model, Ob acts like a switchboard controlled by P.bf. P.bf selects attended odor paths in Ob, where Ob either passes the odor signal to its destination or inhibits the signal if it’s a distractor. P.bf opens and closes gated circuits in Ob.
Although the architecture of the Ob and P.bf circuit resembles the P.ni circuit, Ob appears to rely more heavily on inhibitory GABA for the gating operation, although ACh is also important [Böhm et al 2020], [de Saint Jan et al 2020], [Nunez-Parra et al 2000]. Since this essay is a first cut, simplified model, I’m using a single signal that represents a gating attention / inhibition signal, and glossing over the ACh vs GABA distinction.
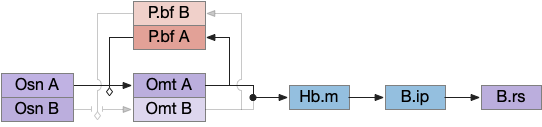
In the above diagram where the switchboard selects odor A and inhibits odor B, the apical seek circuit receives only odor A’s signal. P.bf gates odors from Osn (olfactory sensory neurons) to Omt (mitral/tufted output cells), which then add to form a single signal for the temporal gradient tumble-and-run seek. For simplicity, I’ve shown the P.bf ACh and GABA signal as a simple gating control.
Once the system detects odor A, P.bf configures the switchboard to pass through A and inhibit other odors, locking out the distractor. Because the selecting signals are modulators, they don’t drive a signal until an odor signal arrives. Like the P.ni circuit, attention will timeout as ACh and its slow mACh receptor decay. When the animal leaves the odor plume, the system resets because the absence of odor A collapses the feedback loop.
Although the essay’s switchboard is an improvement over the naive summation of odor signals, it’s still quite limited. There’s no active selection of a best odor, and the system can’t switch to a better odor cue. Also, since the global give-up circuit isn’t integrated with P.bf, giving up on odor A can’t select odor B. Instead the animal must leave the plume and reset the system.
Slightly more complete Ob switchboard
The Ob is a surprisingly complex system; it’s not just a simple odor system. In addition to the P.bf, Opir (olfactory piriform cortex) also modulates the Ob system, and Ob itself has lateral inhibition between Omt (mitral cell output), which is plastic, learning to discriminate odors itself, as well as modulatory input from the serotonin and noradrenaline system.
In the real Ob, many Osn for the same odor feed into a single Ogl (olfactory glomeruli), which provides input to several Omt, all representing the same odor. Each odor feature has its own Ogl system, several hundred in mammals (two in the essay simulation). Ogl is where the neuropil of the Osn axons meet the Omt dendrites, in a fan-in to fan-out system. Also, each Ogl has many inhibitory Opg (periglomerular inhibitors) with multiple variations, and each Omt has several inhibitory Ogc (olfactory granule cells). The basic fan-in and fan-out structure looks like the following diagram.
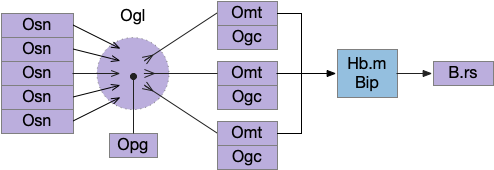
The switchboard diagram below focuses on the ACh and GABA control from P.bf. It combines multiple Osn, Opg, Omt and Ogc into single items.
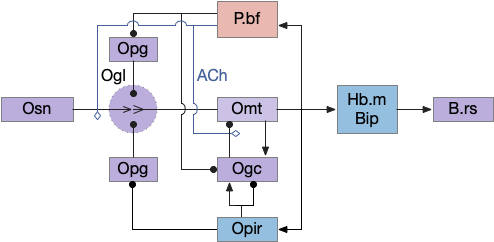
To break down the diagram, the core of the switchboard circuit is the Osn to Ogl to Omt to output path; everything else is gating to select or inhibit the signal.
Odor gating happens in two locations: modulating Omt’s input dendrite tree in Ogl by Opg and modulating Omt’s output by Ogc (olfactory granular cell). Because each Omt’s input Ogl is shared for several Omt, the Opg inhibition likely affects many or all Omt for a single Ogl. In contrast, the Ogc inhibition is individual, and the Omt and Ogc circuit creates and manages gamma oscillations, which amplifies and reduces noise from the signal.
Although I’m not planning on touching cortical areas for many essays, the Opir (olfactory piriform cortex) modules the Ob switchboard in a similar circuit as B.pf with some difference. Since the Opir input to the many Ogc and many Ogl is not odor selective [Boyd et al 2015], Ogc must learn the meaning of the Opir input through plasticity.
Global give-up circuit
The essay’s task engagement and give-up circuit currently uses H.l (lateral hypothalamus) and Hb.l (lateral habenula) with V.dr (dorsal raphe serotonin) [Hikosaka 2010], [Chowdhury and Yamanaka 2016]. When a seek fails Hb.l suppresses H.l, H.l ends seek, and the animal moves on [Post et al 2022].
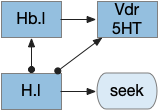
Because the global give-up circuit is entirely disconnected from the olfactory selective attention from the essay, giving up means giving up on all odors, not just the current attended odor.
Simulation
For this essay, I refactored much of the simulation code to clean up ideas from previous essays. A new hindbrain module manages the main locomotion like the zebrafish hindbrain motor area [Dunn et al 2016], which is possibly different from the tetrapod / amniote locomotion in the midbrain. Because the essay animal is currently more primitive than amniotes, this simplification seemed appropriate and makes the code organization more clear.
Olfactory locomotion is now random-walk based following apical tumble-and-run, as opposed to the earlier bilateral path through Vta (ventral tegmental area / posterior tuberculum) and OT (tectum). In zebrafish both paths exist, which I might explore later, but this essay is restricted to the apical temporal gradient search.
The seek mode now slows the animal and adjusts the Levy walk parameters to simulate ARS (area restricted search). As I’ll cover in the problems section, switching to seek mode is still hardcoded.
I split the habenula seek from habenula give-up (Hb.m from Hb.l) and pulled the gradient seek and head direction from B.ip into the habenula seek. Conceptually, the habenula seek code now represents Hb.m and B.ip as a single complex.
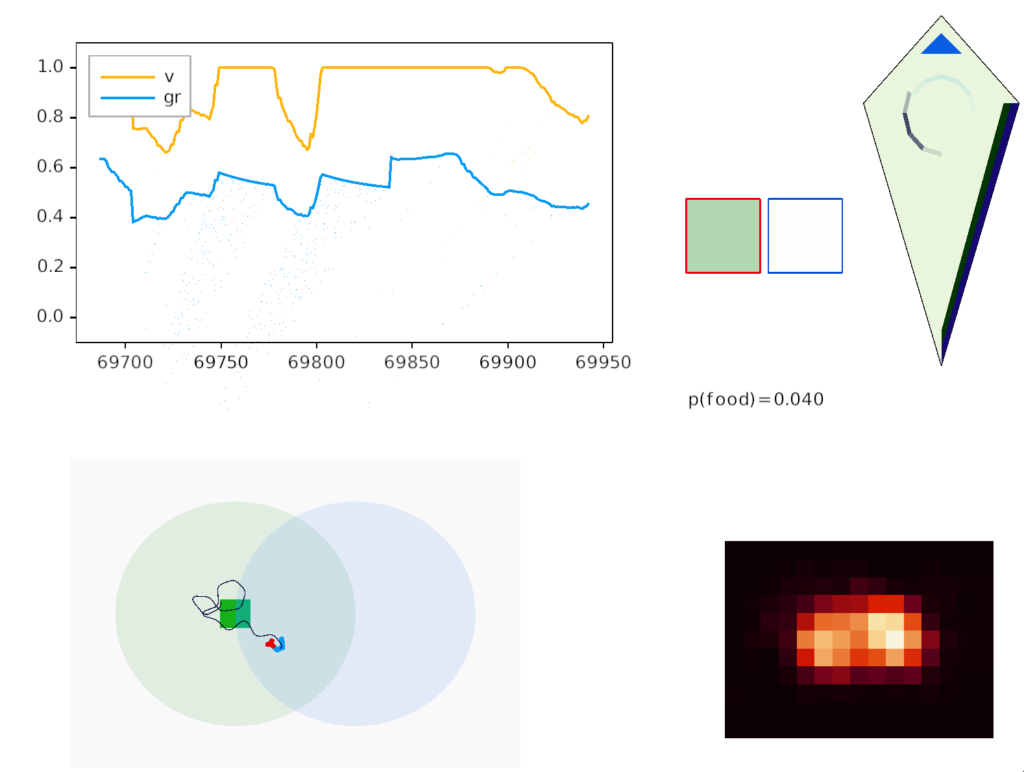
In the screenshot above, the animal is making a u-turn to return to the food when the odor gradient (blue semicircle) is opposite the head direction (black semicircle). In the upper right, the green box outlined in red represents the attended green odor signal, while the white box outline in blue represents the suppressed blue odor. Despite the Osn naively sensing both blue and green odors because the animal is in the overlap area, only the green odor passes through Omt to the seek system.
The square borders around the odor color represent P.bf modulation. Red is attended (100% pass through), blue is inhibited (10% pass through), and grey is unmodulated (50% pass through).
In the diamond-shaped homunculus, the bright blue triangle represents the u-turn nudge.
As the goal vector shows, the guessed goal direction isn’t very accurate, particularly when the animal is making a turn. Currently, the animal continues to update its guess even in the middle of a turn when the odor data and averages are not appropriate for the current direction.
References
Böhm E, Brunert D, Rothermel M. Input dependent modulation of olfactory bulb activity by HDB GABAergic projections. Sci Rep. 2020 Jul 1;10(1):10696.
Boyd AM, Kato HK, Komiyama T, Isaacson JS. Broadcasting of cortical activity to the olfactory bulb. Cell Rep. 2015 Feb 24;10(7):1032-9.
Braitenberg, V. (1984). Vehicles: Experiments in synthetic psychology. Cambridge, MA: MIT Press. “Vehicles – the MIT Press”
Chowdhury S, Yamanaka A. Optogenetic activation of serotonergic terminals facilitates GABAergic inhibitory input to orexin/hypocretin neurons. Sci Rep. 2016;6:36039
Cisek P. Evolution of behavioural control from chordates to primates. Philos Trans R Soc Lond B Biol Sci. 2022 Feb 14
De Saint Jan D. Target-specific control of olfactory bulb periglomerular cells by GABAergic and cholinergic basal forebrain inputs. Elife. 2022 Feb 28;11:e71965.
Dunn, Timothy, Yu Mu, Sujatha Narayan, Owen Randlett, Eva A Naumann, Chao-Tsung Yang, Alexander F Schier, Jeremy Freeman, Florian Engert, Misha B Ahrens (2016) Brain-wide mapping of neural activity controlling zebrafish exploratory locomotion eLife 5:e12741
Henriques PM, Rahman N, Jackson SE, Bianco IH. Nucleus Isthmi Is Required to Sustain Target Pursuit during Visually Guided Prey-Catching. Curr Biol. 2019 Jun 3;29(11):1771-1786.e5.
Hikosaka O. The habenula: from stress evasion to value-based decision-making. Nat Rev Neurosci. 2010 Jul;11(7):503-13.
Nunez-Parra A, Cea-Del Rio CA, Huntsman MM, Restrepo D. The Basal Forebrain Modulates Neuronal Response in an Active Olfactory Discrimination Task. Front Cell Neurosci. 2020 Jun 5;14:141.
Post RJ, Bulkin DA, Ebitz RB, Lee V, Han K, Warden MR. Tonic activity in lateral habenula neurons acts as a neutral valence brake on reward-seeking behavior. Curr Biol. 2022 Oct 24;32(20):4325-4336.e5.
Tosches, Maria Antonietta, and Detlev Arendt. The bilaterian forebrain: an evolutionary chimaera. Current opinion in neurobiology 23.6 (2013): 1080-1089.